Q1. Discuss different types of salt hydrolysis.
Solution
Salts of following types exhibit the phenomena of salt hydrolysis.
In salts of weak acids and strong bases anions get hydrolyzed to give more of OH- and the solution is alkaline. It is called anionic hydrolysis.
In salts of strong acids and weak bases Cations get hydrolyzed to give more of H+ and the solution is acidic. It is called cationic hydrolysis.
In salts of weak acids and weak bases both anions and cations get hydrolyzed the acidity or basicity of the solution depends upon Ka and kb. When ka = kb the solution is neutral. It is called anionic hydrolysis.
Q2. Define ionization Constant? Show how can we derive expression for Ka for a weak acid like CH3COOH?
Solution
Ionization constant is the product of Equilibrium constant K and concentration of water term in an ionic equilibrium of a weak electrolyte.
We can derive an expression from the following ionic equilibrium of CH3COOH in water.
CH3COOH + H2O  CH3COO- + H+
Initial molar concentration C 0 0
Molar conc. at equilibrium point C(1 – α ) c α c α
Applying the law of chemical equilibrium
K =
[CH3COO-] [H+]
CH3COO- + H+
Initial molar concentration C 0 0
Molar conc. at equilibrium point C(1 – α ) c α c α
Applying the law of chemical equilibrium
K =
[CH3COO-] [H+]  [CH3COOH] [H2O]
K [H2O] =
cα2
[CH3COOH] [H2O]
K [H2O] =
cα2  (1–α )
cα2 Or, Ka =
(1–α )
cα2 Or, Ka =  (1 – α )
Where K is equilibrium constant and Ka is known as ionization or dissociation constant of the acid, similarly a Kb expression can be derived for a weak base.
(1 – α )
Where K is equilibrium constant and Ka is known as ionization or dissociation constant of the acid, similarly a Kb expression can be derived for a weak base.
 CH3COO- + H+
Initial molar concentration C 0 0
Molar conc. at equilibrium point C(1 – α ) c α c α
Applying the law of chemical equilibrium
K =
[CH3COO-] [H+]
CH3COO- + H+
Initial molar concentration C 0 0
Molar conc. at equilibrium point C(1 – α ) c α c α
Applying the law of chemical equilibrium
K =
[CH3COO-] [H+]
Q3. How will you differentiate between physical and chemical equilibria?
Solution
Physical equilibria do not involve changes to the chemical properties of the substances involved. For instance, equilibrium between water vapor and liquid water in a partly filled sealed container. It is a physical equilibrium since the water molecules have only changed from liquid to vapor. Chemical equilibria on the other hand involve changes in the chemical composition of substances. Bond breaking and bond formation is involved. An example would be the dissociation of acetic acid. There is an interchange of particles between ions and molecules whose rate of exchange becomes equal when equilibrium is established. CH3COOH + H2O  H3O +1 + CH3COO-1
H3O +1 + CH3COO-1
 H3O +1 + CH3COO-1
H3O +1 + CH3COO-1
Q4. Calculate the dissociation constant of a monobasic acid having the degree of dissociation 2% in its 0.01M solution.
Solution
Use Ionization constant expression
 Substitute the respective values; we get
Ka = (0.01)(0.02 x 0.02)/ (1 - 0.02)
= 4.08 x 10-6 M/L
Substitute the respective values; we get
Ka = (0.01)(0.02 x 0.02)/ (1 - 0.02)
= 4.08 x 10-6 M/L
 Substitute the respective values; we get
Ka = (0.01)(0.02 x 0.02)/ (1 - 0.02)
= 4.08 x 10-6 M/L
Substitute the respective values; we get
Ka = (0.01)(0.02 x 0.02)/ (1 - 0.02)
= 4.08 x 10-6 M/L
Q5. Calculate the value of Ka for the following reaction having the equilibrium concentration of H+ 7.1 x 10-5 mol/L in a 0.05 molar solution. H2S  H+ + HS-
H+ + HS-
 H+ + HS-
H+ + HS-Solution
[H+] = C?
Or, ? = 7.1 x 10-5/ 0.05 = 1.42 x 10-3
Ka = C ?2
= .05 x 1.42 x 10-3 x 1.42x 10-3 = 1.0 x 10-7
Q6. The Ksp for Ag2CrO4 is 9x10-12 M3. What is the molar solubility of Ag2CrO4 in pure water?
Solution
Let x be the molar solubility of Ag2CrO4, then
Ag2CrO4 = 2 Ag+ + CrO42-
2 x x
(2 x)2 (x) = Ksp
x = {(9x10-12)/4}(1/3)
= 1.3x10-4 M
Hence, the molar solubility of Ag2CrO4 is 1.3x10-4 M.
Q7. What is the concentration of barium and sulfate ions in a saturated barium sulfate solution at 25°C?
Solution
Ksp = [Ba2+][SO42-] = 1.1 x 10-10
In this reaction,
BaSO4(s) <---> Ba2+(aq) + SO42-(aq)
[Ba2+] = [SO42-]
So we can substitute one concentration for the other!
[Ba2+][Ba2+]=[Ba2+] 2 = 1.1 x 10-10
[Ba2+] = 1.05 x 10-5
Q8. Explain that chemical equilibria can be achieved from either side.
Solution
Q9. A crystal of common salt of given mass is kept in aqueous solution. After 12 hours, its mass remains the same. Is the crystal in equilibrium with the solution?
Solution
Yes, as the solution becomes saturated the mass of the crystal remains same because of establishment of a physical equilibrium between the crystal of the common salt and its saturated solution.
Q10. Give the conjugate acid for each of the following: OH-, CH3COO-, Cl-, CO3-2, CH3NH2
Solution
The conjugate acids are respectively as below:
H2O, CH3COOH, HCl , HCO3-1 CH3NH3+
Q11. The pOH of a solution of NaOH is 11.00. What is the [H+] for this solution?
Solution
pOH = 14 - pH
11 = 14 - pH
pH = 14 - 11 = 3
pH = - log [H+]
-3 = log [H+]
[H+] =Anti [- 3]
[H+] = 0.001
Q12. Chemical equilibrium is dynamic in nature. Explain.
Solution
A chemical equilibrium is dynamic in nature which in other words means that reactions continue to occur in both forward as well as backward direction with the same speed. As a result, the amount of product formed immediately reacts backward to give reactants and so there is no change in concentration of reactant or product with the passage of time.
Q13. The calculated value of Kb of NH3 is 1.8 x 10-5. Calculate the pH of 1.5 M solution of ammonia.
Solution
Q14. For the equilibrium system described by 2 SO2 (g) + O2 (g) ↔ 2 SO3 (g) at a particular temperature the equilibrium concentrations of SO2, O2 and SO3 were 0.75 M, 0.30 M, and 0.15 M, respectively. Calculate the equilibrium constant, Keq, for the reaction.
Solution
Equilibrium constant expression for the balanced equation:
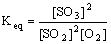 substitute the known values, and solve for the unknown Keq
substitute the known values, and solve for the unknown Keq
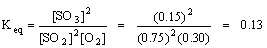
Q15. The pH of lemon juice is 2.32 at 298 k. What will be its [H3O+]?
Solution
pH = -log [H3O+]
2.32 = -log [H3O+]
antilog (- 2.32) = [H3O+]
[H3O+] = 4.786 x 10-3
[OH-] = 10-14 / 4.786 x 10-3 = 2.09 x 10-12
Q16. The following reaction is a reversible reaction. The value of Kc for this reaction is 31.4 at 588K.
CO(g) + H2O (g)  CO2 (g) + H2 (g) If a 10.00L vessel has 2.50 mol CO2 and H2O, and 5.00 mol CO2 and H2 gas at 588K, which way will the reaction proceed ?
CO2 (g) + H2 (g) If a 10.00L vessel has 2.50 mol CO2 and H2O, and 5.00 mol CO2 and H2 gas at 588K, which way will the reaction proceed ?
 CO2 (g) + H2 (g) If a 10.00L vessel has 2.50 mol CO2 and H2O, and 5.00 mol CO2 and H2 gas at 588K, which way will the reaction proceed ?
CO2 (g) + H2 (g) If a 10.00L vessel has 2.50 mol CO2 and H2O, and 5.00 mol CO2 and H2 gas at 588K, which way will the reaction proceed ?Solution
[CO] = .25 M
[H2O] = .25 M
[CO2] = .50 M
[H2] =.50 M
Hence, Qc = [CO2] [H2]/ [CO] [H2O] = 4
Since Qc < Kc, it will proceed in the forward direction.
Q17. An aqueous solution of Sr(NO3)2 is added slowly to 1.0 liter of a well-stirred solution containing 0.020 mole F- and 0.10 mole SO42- at 25 °C( ΔV = O) Which salt precipitates first? What is the concentration of strontium ion (Sr2+), in the solution when the first precipitate begins to form? The solubility product constant, Ksp for strontium sulfate, SrSO4, is 7.6 x 10-7. The solubility product constant for strontium fluoride, SrF2, is 7.9 x 10-10
Solution
Calculate [Sr2+] required for precipitation in each salt
Case-1
Let the [Sr2+] be x
Ksp = [Sr2+][F-]2 = 7.9 x 10 -10
= (x) (0.020 mole / 1.0 L)2 = 7.9 x 10-10
x = 2.0 x 10-6 M
Case-2
Let the [Sr2+] be y
Ksp = [Sr2+][SO42-¯] = 7.6 x 10-7
= (y) (0.10 mole/1.0 liter) = 7.6 x 10-7
y = 7.6 x 10-6 M
Since 2.0 x 10-6 M < 7.6 x 10-6 M,
SrF2 must precipitate first.
When SrF2 precipitates, [Sr2+] = 2.0 x 10-6 M
Q18. The Ksp for AgCl is 1.8 x 10-10. If Ag+ and Cl- are both in solution and in equilibrium with AgCl. What is [Ag+] if [Cl-] = .020 M?
Solution
[Ag+] = Ksp /[Cl-]
= 1.8 x 10-10/.020
[Ag+] = 9.0 x 10-9M
Q19. A solution is formed by combining 0.025 mol CaCl2 and 0.015 mol Pb(NO3)2 producing one litre of solution. Will PbCl2 precipitate in this solution?
Solution
0.015 mol of Pb(NO3)2 gives 0.015 mol of Pb2+ ions or ,0.015 M)
0.025 mol of CaCl2 yields 0.05 mol Cl- ions or. 0.05 M
Hence , [Pb2+][Cl]2 = (0.015)(0.050)2 = 3.75 x 10-5
Ksp of PbCl2 = 1.6 x 10-5
Since the ion product is larger than the Ksp, the solution precipitates.
Q20. CO2 gas is more soluble in aq. NaOH solution than in water, explain.
Solution
CO2 dissolves in water to form carbonic acid (H2CO3). As their reaction is reversible carbonic acid (H2CO3) dissociates to give CO2 and H2O in the backward reaction.
However, with aqueous NaOH solution , H2CO3 reacts as below:
H2CO3(aq) + NaOH(aq) → Na2CO3(aq) + H2O
As a result, more of CO2 dissolves in water to form carbonic acid. This means that the gas is more soluble in aqueous NaOH than in water.
Q21. A solution is made by dissolving 0.63 gm of nitric acid in 200 ml of water, assuming that the acid is completely dissociated, calculate its pH value.
Solution
Molarity of the solution = w/m/V in litre = 0.63/63/.2 = .05 M
Hence [H3O+] = 5.0 X 10-2 (assuming that the acid is completely dissociated)
pH = - log[H3O+]
= 1.30
Q22. The following reaction is at equilibrium: 4NH3 (g) + 3O2 (g)  6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added
6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added
 6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added
6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is addedSolution
a) Since the volume is being increased, the pressure is dropping. In response, the equilibrium will shift right to attempt to make more moles of gas and increase the pressure.
b) The equilibrium will not change. To change the gaseous equilibrium, the partial pressures of at least one gas need to be changed. Since He is un-reactive with all of the gases present, the total pressure will change but the partial pressures will not be affected.
Q23. What is the common ion effect?
Solution
The solubility of slightly soluble substances can be decreased by the presence of a common ion. This effect is known as common ion effect.
Addition of a common ion to a slightly soluble salt solution will add up to the concentration of the common ion. According to Le Chatelier's Principle that will place a stress upon the slightly soluble salt equilibria. Thus, the equilibrium will shift so as to undo the stress of added common ion.
Q24. The ionization constant of propionic acid is 1.32 x 10-5. Calculate the degree of ionization of acid in its 0.05 M solution.
Solution
Use Oswald’s Dilution Law expression
α = √ ka/C
Substitute the respective values; we get
α = √ 1.32x 10-5/0.05
= 16.24 X10-2
= 0.16
=16%
Q25. Equal volumes of 0.1 M MgSO4 and 0.1 M Ba Cl2 are mixed together. Predict the formation of BaSO4 precipitate. Given Ksp of BaSO4 = 1.5 x 10-9
Solution
BaSO4  Ba2+ + SO4-
Ksp = [Ba2+][ SO4-2] = 1.5 x 10-9
Concentration of[Ba2+]and [ SO4-2] in the final solution
[Ba2+] = 0.1 M/2 = 0.05 M
[ SO4-2] = 0.1 M/2 = 0.05 M
Now Ionic product = 0.05 x 0.05 = 2.5 x 10 -3 which is greater than Ksp = [Ba2+][ SO4-2] = 1.5 x 10-9
Hence the precipitation of BaSO4 will occur.
Ba2+ + SO4-
Ksp = [Ba2+][ SO4-2] = 1.5 x 10-9
Concentration of[Ba2+]and [ SO4-2] in the final solution
[Ba2+] = 0.1 M/2 = 0.05 M
[ SO4-2] = 0.1 M/2 = 0.05 M
Now Ionic product = 0.05 x 0.05 = 2.5 x 10 -3 which is greater than Ksp = [Ba2+][ SO4-2] = 1.5 x 10-9
Hence the precipitation of BaSO4 will occur.
 Ba2+ + SO4-
Ksp = [Ba2+][ SO4-2] = 1.5 x 10-9
Concentration of[Ba2+]and [ SO4-2] in the final solution
[Ba2+] = 0.1 M/2 = 0.05 M
[ SO4-2] = 0.1 M/2 = 0.05 M
Now Ionic product = 0.05 x 0.05 = 2.5 x 10 -3 which is greater than Ksp = [Ba2+][ SO4-2] = 1.5 x 10-9
Hence the precipitation of BaSO4 will occur.
Ba2+ + SO4-
Ksp = [Ba2+][ SO4-2] = 1.5 x 10-9
Concentration of[Ba2+]and [ SO4-2] in the final solution
[Ba2+] = 0.1 M/2 = 0.05 M
[ SO4-2] = 0.1 M/2 = 0.05 M
Now Ionic product = 0.05 x 0.05 = 2.5 x 10 -3 which is greater than Ksp = [Ba2+][ SO4-2] = 1.5 x 10-9
Hence the precipitation of BaSO4 will occur.
Q26. For the equilibrium system described by:
PCl5 (g)  PCl3 (g) + Cl2 (g) Keq equals 35 at 487°C. If the concentrations of the PCl5 and PCl3 are 0.015 M and 0.78 M, respectively, what is the concentration of the Cl2?
PCl3 (g) + Cl2 (g) Keq equals 35 at 487°C. If the concentrations of the PCl5 and PCl3 are 0.015 M and 0.78 M, respectively, what is the concentration of the Cl2?
 PCl3 (g) + Cl2 (g) Keq equals 35 at 487°C. If the concentrations of the PCl5 and PCl3 are 0.015 M and 0.78 M, respectively, what is the concentration of the Cl2?
PCl3 (g) + Cl2 (g) Keq equals 35 at 487°C. If the concentrations of the PCl5 and PCl3 are 0.015 M and 0.78 M, respectively, what is the concentration of the Cl2?Solution
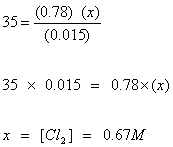
Q27. Calculate the concentration of hydroxyl ion in 0.2 M solution of NH3OH having Kb = 1.8 x 10-4.
Solution
Use Oswald’s Dilution Law formula;
α = √ kb/C
Substitute the respective values; we get
α =√ kb/C = √1.8 x 10-4 /0.2 = .03 = 3%
[OH-] = 0.2 x .03 = 6.0 x 10 -3 M
Comments
Post a Comment