Q1. The spatial orientation of sp3d3 hybrid orbitals is:
Solution
The spatial arrangement of sp3d3 hybrid orbitals is pentagonal bi pyramidal.
Q2. Calculate the formal charge on the following: a) O atoms of O3 b) Cl atom in HClO4 ion c) S in HSO4- ion
Solution
Q3. In which of the following molecules bond angle is the largest?
Solution
Molecule
Hybridization
Geometry
Bond angle
CO2
sp
Linear
180°
CH4
sp3
Tetrahedral
109°.5
NH3
sp3
Trigonal Pyramidal
107°.5
CCl4
sp3
Tetrahedral
109°.5
Q4. Two p-orbitals from one atom and two p-orbitals from other atom are combined to form molecular orbitals. How many molecular orbitals will result from this combination? Explain.
Solution
Four molecular orbitals are formed when two p-orbitals from one atom combines with the two p-orbitals of the other atom. Out of these four molecular orbitals, two are bonding molecular orbitals and the other two are antibonding molecular orbitals.
Q5. (i) Describe the conditions necessary for hydrogen bonding. (ii) Differentiate between intermolecular and intramolecular hydrogen bonding. (iii) How does energy of the conical structures contribute to the stability of resonance hybrid?
Solution
(i) The two conditions that are necessary for hydrogen bonding are: (a) Hydrogen atom should be bonded to a highly electronegative atom. (b) The size of the electronegative atom should be small.(ii)
Intermolecular hydrogen bonding
Intramolecular hydrogen bonding
(i) It is formed between two different molecules of the same or different substances.
(i) It is formed between the hydrogen atom and highly electronegative atom present within the same molecule.
(ii) Example: HF, alcohol, water molecule.
(ii) Example: o-nitro phenol, o-nitro benzoic acid.
(iii) The canonical structures of similar energy contribute equally to the resonance hybrid whereas the structure with higher energy is less stable and has lesser contribution to the resonance hybrid.
Q6. Explain why sucrose is quite soluble in water though it is a covalent compound.
Solution
A molecule of sucrose contains many –OH groups and hence is capable of forming H- bond with water. As a result of this it is soluble in water.
Q7. "BeH2 molecule has zero dipole moment although the Be-H bonds are polar." Explain this sentence on the basis of the concept of dipole moment.
Solution
The BeH2 molecule is linear with (H-Be-H) bond angle equal to 180o.
Although the Be-H bonds are polar due to the difference in the electronegativities of Be and H atom, the bond polarities cancel each other.
As a result the resultant dipole moment is zero.
Q8. A diatomic molecule has dipole moment of 1.92D and bond length of 2A. Calculate the percentage ionic character of the molecule.
Solution
μ = q x d
= 4.8 x 10-10 esu x 1.2 x 10-8 cm
= 5.76 x 10-18 esu. cm = 5.76D
% ionic character = 1.92 D x 100 = 33.3%
5.76 D
Q9. Calculate the bond order of the following molecules/ions: H2+, N2, CN, CO. State their magnetic behavior.
Solution
Q10. Draw the molecular orbital diagram of N2. Also find its bond order and magnetic character?
Solution
Molecular orbital diagram of N2 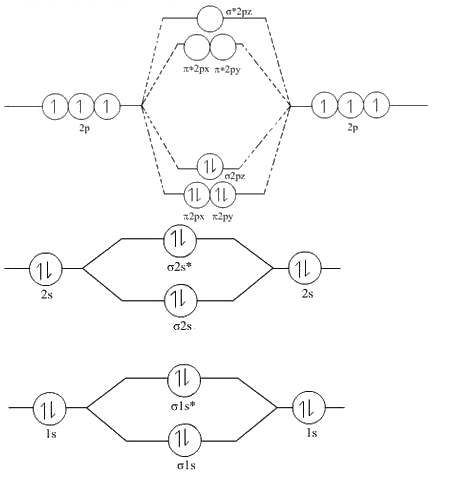 BO =
BO = 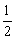 [Nb-Na] =
[Nb-Na] = 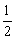 [10-4] = 3Since all the electrons in nitrogen are paired, it is diamagnetic molecule.
[10-4] = 3Since all the electrons in nitrogen are paired, it is diamagnetic molecule.
 BO =
BO =
Q11. Define hybridization. Show the formation of ammonia (NH3) molecule.
Solution
i) It can be defined as the process of intermixing of the orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of new set of orbitals of equivalent energies and shape.
ii) Formation of ammonia molecule:
In ammonia molecule, the nitrogen atom is sp3 hybridized.
Three sp3-hybrid orbitals of N atom are used for forming sp3-s 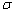 (sigma) bonds with H atoms.
The fourth sp3-hybrid orbital carry lone pair of electrons.
The relatively larger lp-bp interactions cause H-N-H angle to decrease from 109o 28' to 107o.
(sigma) bonds with H atoms.
The fourth sp3-hybrid orbital carry lone pair of electrons.
The relatively larger lp-bp interactions cause H-N-H angle to decrease from 109o 28' to 107o.

Q12. The bond angles of PF3, PCl3, PBr3 and PI3 are 97o, 100o, 101.5o and 102o respectively. This data shows a gradual increase in the bond angle. Explain.
Solution
As the electronegativity of surrounding atom decreases, the electron pair lie closer to the central atom so the repulsion between the electrons increases and the bond angle also increases.
Q13. Discuss the factors on which the magnitude of bond energy depends.
Solution
Bond energy depends on:
Size of the participating atom: Larger the size of participating atom, smaller is the value of bond energy. The large size of the atom allows lesser extent of orbital overlapping.
Multiplicity of bond: The magnitude of bond energy increases with the multiplicity of bond, even though the atoms involved would be the same. This is because the number of shared electrons increases with the multiplicity of bonds.
Q14. Draw the Lewis structures of SiCl4 and HCOOH.
Solution
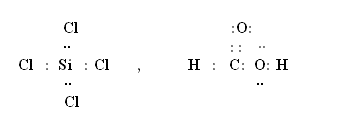
Q15. Explain why helium molecule does not exist?
Solution
Helium atom (1s2) has 2 electrons in its 1s orbital.
During the approach of two helium atoms, the repulsive forces are dominant over the attractive forces. As a result the energy of the system increases which leads to instability.
Since energy of the separate helium atoms is smaller than that of the system when they are close to each other, they prefer to stay separate and do not form He2 molecule.
Q16. Draw an accurate Lewis structure for HONO2, including all non-bonded electron pairs and formal charges. Additionally, provide bond angles around the nitrogen atom.
Solution
The structure of HNO3 is: 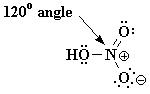

Q17. What do you understand by the term valence electrons?
Solution
The electrons present in the outershell of an atom that take part in a chemical reaction are known as valence electrons.
Q18. Among H2+ and H2- molecular ions which ion is more stable and why?
Solution
Among H2+ and H2- molecular ions, H2+ ion is more stable because the electron in H2+ is present only in bonding orbital.
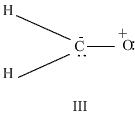
Q19. i)Out of the following resonating forms of formaldehyde which one is least significant and why? 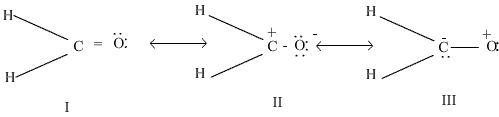 ii) What are the factors on which the dipole moment of polyatomic molecules depends?
ii) What are the factors on which the dipole moment of polyatomic molecules depends?
 ii) What are the factors on which the dipole moment of polyatomic molecules depends?
ii) What are the factors on which the dipole moment of polyatomic molecules depends?Solution
i) Among the given structures the III structure is least significant because here positive charge is present on more electronegative atom.
 ii) The dipole moment of polyatomic molecules depends upon the individual dipole moments of the bonds and the spatial arrangement of various bonds of the molecules.
ii) The dipole moment of polyatomic molecules depends upon the individual dipole moments of the bonds and the spatial arrangement of various bonds of the molecules.
 ii) The dipole moment of polyatomic molecules depends upon the individual dipole moments of the bonds and the spatial arrangement of various bonds of the molecules.
ii) The dipole moment of polyatomic molecules depends upon the individual dipole moments of the bonds and the spatial arrangement of various bonds of the molecules.
Q20. Draw the resonating structures of nitrate ion. Calculate the bond order of N-O.
Solution
The resonating structures of nitrate ion NO3- are: 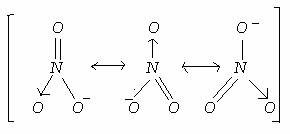 Bond order of NO bond is = 4/3 = 1.33
Bond order of NO bond is = 4/3 = 1.33
 Bond order of NO bond is = 4/3 = 1.33
Bond order of NO bond is = 4/3 = 1.33
Q21. Draw the shapes of AB2E2 and AB2E3 type of molecules. Also mention the number of bond pairs, lone pair of electrons and examples of the molecules.
Solution
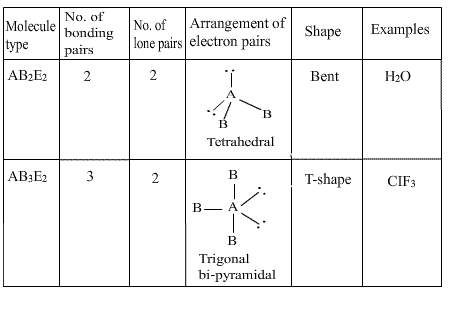
Q22. The elements of the third period of the periodic table have expanded octet. Explain.
Solution
The elements present in the third period and beyond it have 3d orbitals apart from 3s and 3p orbitals for bonding.
So in number of compounds of these elements there are more than eight valence electrons around the central atom.This is known as expanded octet.
Q23. What is lattice energy? Which compound NaCl or MgO has higher lattice energy and why?
Solution
The Lattice Enthalpy of an ionic solid is defined as the energy required to completely separate one mole of a solid ionic compound into gaseous constituent ions.Lattice energy depends on:
Size of the ions: Small is the size of ion, lesser is the inter-nuclear distance and greater will be the attraction, thus larger will be the magnitude of lattice energy.
Charge on the ions: More the charge on the ion, higher is the lattice energy.
Between NaCl and MgO, lattice energy of MgO is higher as the positive charge on Mg is higher than Na ion.
Q24. Which has higher boiling point o-nitro phenol or p-nitro phenol? Give reason for your answer.
Solution
p-nitrophenol has higher boling point than o-nitrophenol.
This is due to intermolecular hydrogen bonding i.e. p-nitrophenol molecules can associate with themselves.
o-nitrophenol does not undergo intermolecular hydrogen bonding because of chelation.
Q25. Using Molecular Orbital theory, compare the bond enthalpy and bond length of CN and CN- species.
Solution
Number of electron in CN = 6+7=13
MO configuration of CN
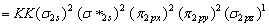 B.O =
B.O =  [7-2] = 2.5
MO configuration of CN-
[7-2] = 2.5
MO configuration of CN-
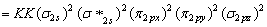 BO =
BO = 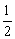 (8-2) = 3
Higher the bond order greater is the bond energy and shorter is the bond length. Hence bond enthalpy of CN- is greater than CN and bond length of CN- is shorter than the bond length of CN.
(8-2) = 3
Higher the bond order greater is the bond energy and shorter is the bond length. Hence bond enthalpy of CN- is greater than CN and bond length of CN- is shorter than the bond length of CN.
Q26. "In the molecule H-Br the H would have a partial negative charge and the Br would have a partial positive charge." Is it true? Explain.
Solution
No, the statement is wrong.
In HBr, the H has partial positive charge while Br has partial negative charge.
Br being more electronegative attracts the shared pair of electrons towards itself.
The compound HBr is a polar covalent compound.
Q27. Explain why reactions involving covalent compounds are generally slow?
Solution
Covalent bond compounds are formed by the mutual sharing of electrons between the atoms in the compound. These compounds do not produce ions in the aqueous solution.
Reactions of covalent bonded compounds involve breaking of bonds in reacting molecules and forming new covalent bonds in the products. Thus, these reactions are relatively slow.
Q28. Compare three types of bonding in terms of bond energy and directional characteristics.
Solution
Type of Bond
Bond Energy
Other characteristics
Ionic
strong
Non-directional
Covalent
variable
Directional
Metallic
variable
Non-directional
Q29. Ionic compounds do not conduct electricity when solid. Why? When do they conduct electricity?
Solution
Ionic compounds are formed by attraction between the positive and negative ions. These ions cannot move out of their fixed positions. Thus, solid ionic compounds do not conduct electricity.
When ionic compounds are in molten state or dissolved in water forming a solution, the ions can move. Then ionic compounds conduct electricity.
Q30. MgCl2 is a linear molecule while SnCl2 is angular.Explain.
Solution
In MgCl2, the central atom i.e. the magnesium ion is surrounded by only two bond pairs so there is no repulsion and the compound posses a regular geometry whereas in case of SnCl2 molecule the central atom is surrounded by two bond pairs and two lone pairs of electrons which leads to repulsion among them as a result the molecule posses distorted geometry.
Q31. Explain why the bond order of N2 is greater than N2+ but the bond order of O2 is less than that of O2+.
Solution
When N2 changes to N2+, the electron is removed from the bonding molecular orbital while when O2 changes to O2+, the electron is removed from antibonding molecular orbital. This is the reason why the bond order of N2 is greater than N2+ but the bond order of O2 is less than that of O2+.
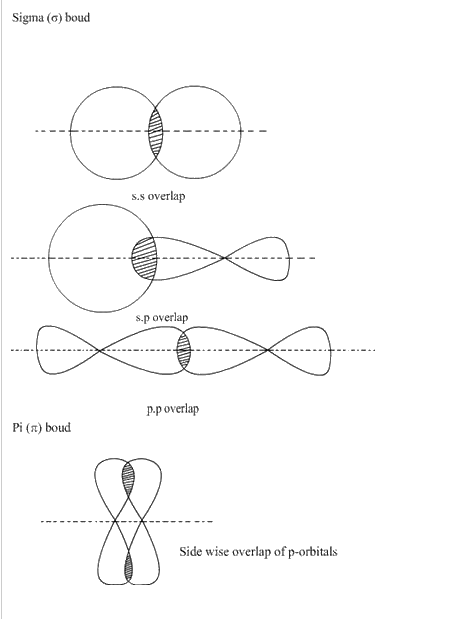
Q32. Explain the formation of sigma and pi bond.
Solution
Sigma bond is formed by the axial overlapping of half filled atomic orbitals where as pi bond is formed by the lateral or sidewise over lap of the atomic orbitals.

Q33. Among the following structures of SF4 which one is more stable and why? What is this shape called? 

Solution
In figure (a), the lone pair of electrons is present at the axial position so there are three lone pair-bond pair repulsions at 90o. In the figure(b), the lone pair of electrons is present at the equatorial position and there are two lone pair- bond pair repulsions. Hence figure (b) is more stable as compared to figure (a). The shape shown in figure (b) is distorted tetrahedron, a folded square or a see saw.
Q34. Why the energy levels of molecular orbitals of B2, C2 and N2 are different?Explain.
Solution
The combination of atomic orbitals takes place due to orbital-orbital interactions.
Due to these interactions the energy level diagram gets modified. In case of atoms like boron, carbon and nitrogen the energy difference of 2s and 2p levels is quite small.
Due to close proximity of 2s and 2p orbitals, the 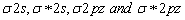 orbitals undergo mixing interactions in view of which the energy of
orbitals undergo mixing interactions in view of which the energy of 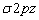 orbitals is raised and it becomes greater than
orbitals is raised and it becomes greater than 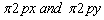 which do not experience these intermixing interactions.
The molecules O2 and F2 do not exhibit these mixing interactions due to larger difference of energy between their 2s and 2pz orbitals which makes intermixing interactions insignificant.
which do not experience these intermixing interactions.
The molecules O2 and F2 do not exhibit these mixing interactions due to larger difference of energy between their 2s and 2pz orbitals which makes intermixing interactions insignificant.
Q35. Define octet rule. What is its significance?
Solution
Atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells. This is known as octet rule.
It is quite useful in explaining the normal valency of large number of elements.
Q36. A compound A has 4 pairs of electrons around the central atom and makes an angle of 109o28'. Draw the shape of the compound.
Solution
The compound has 4 pairs of electrons around the central atom and makes an angle of 109o28', so it is tetrahedral in shape. 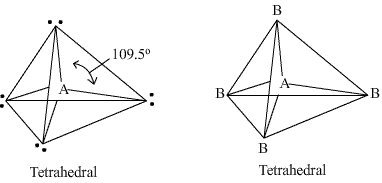

Comments
Post a Comment