Q1. The enthalpies of all elements in their standard states are
Solution
The enthalpies of all elements in their standard states are zero.
Q2. State the effect of the following processes on the total energy content of the system (i) Work done by the system (ii) Heat transferred to the surroundings
Solution
i) Work done by the system is negative i.e. the total energy content of the system decreases. ii) When heat is transferred to the surroundings the total energy content of the system decreases.
Q3. State the condition in which the change in the internal energy of a system ( ) is equal to the heat exchanged q.
) is equal to the heat exchanged q.
 ) is equal to the heat exchanged q.
) is equal to the heat exchanged q.Solution
In a system,  = q when no work is done at constant volume.
= q when no work is done at constant volume.
 = q when no work is done at constant volume.
= q when no work is done at constant volume.
Q4. A gas is compressed by an average pressure of 0.50 atm to decrease its volume from 400 cm3 to 200cm3 . Calculate the amount of work done in this process.
Solution
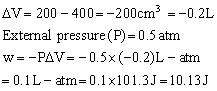
Q5. For a reaction,
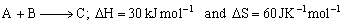 Determine the temperature above which reaction will be spontaneous.
Determine the temperature above which reaction will be spontaneous.
Solution
Q6. Calculate the internal energy of vaporization for 18 g of water at 100°C. Given, 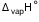 for water at 373 K = 40.66 kJ mol-1.
for water at 373 K = 40.66 kJ mol-1.
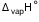 for water at 373 K = 40.66 kJ mol-1.
for water at 373 K = 40.66 kJ mol-1.Solution
The reaction can be represented as follows
18g H2O (l) 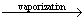 18 g H2O(g)
18 g H2O(g)
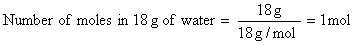
 Assuming steam behaves as an ideal gas
Assuming steam behaves as an ideal gas
 = 40.66 kJ mol -1 – (1) ( 8.314 J K-1mol-1)( 373 K) ( 10-3 kJ J-1)
= 40.66 kJ mol -1 – (1) ( 8.314 J K-1mol-1)( 373 K) ( 10-3 kJ J-1)
 = 40.66 kJ mol -1 – 3.10 kJ mol -1
= 37.56 kJ mol -1
= 40.66 kJ mol -1 – 3.10 kJ mol -1
= 37.56 kJ mol -1
 Assuming steam behaves as an ideal gas
Assuming steam behaves as an ideal gas
 = 40.66 kJ mol -1 – (1) ( 8.314 J K-1mol-1)( 373 K) ( 10-3 kJ J-1)
= 40.66 kJ mol -1 – (1) ( 8.314 J K-1mol-1)( 373 K) ( 10-3 kJ J-1)
 = 40.66 kJ mol -1 – 3.10 kJ mol -1
= 37.56 kJ mol -1
= 40.66 kJ mol -1 – 3.10 kJ mol -1
= 37.56 kJ mol -1
Q7. For a water gas reaction at 1000°C the standard Gibb’s energy change is -8.1 kJ mol-1. Calculate the value of equilibrium constant? 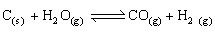
Solution
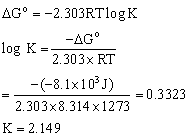
Q8. Give reasons for the following (i) The solubility of most salt in water increases with rise of temperature. (ii) Fluorides tend to be less soluble than corresponding chlorides.
Solution
(i) The solubility of most salt in water increases with rise of temperature because for most of the ionic compounds,  is positive and the dissociation process is endothermic.
(ii) Fluorides tend to be less soluble than corresponding chloride because in former lattice enthalpy is very high.
is positive and the dissociation process is endothermic.
(ii) Fluorides tend to be less soluble than corresponding chloride because in former lattice enthalpy is very high.
 is positive and the dissociation process is endothermic.
(ii) Fluorides tend to be less soluble than corresponding chloride because in former lattice enthalpy is very high.
is positive and the dissociation process is endothermic.
(ii) Fluorides tend to be less soluble than corresponding chloride because in former lattice enthalpy is very high.
Q9. Identify Open, closed or nearly isolated systems from the following. Earth, human beings, can of tomato soup, ice cube tray filled with water, satellite in orbit, coffee in thermos flask, helium filled balloon.
Solution
Earth
Open system
Human beings
Open system
Can of tomato soup
Closed system
Ice cube tray filled with water
Open system
Satellite in orbit
Open system
Coffee in thermos flask
Isolated system
Helium filled balloon
Closed system
Q10. Give the relation between enthalpy of solution, lattice enthalpy and enthalpy of hydration.
Solution

Q11. Calculate the entropy of vaporization of water if its enthalpy of vaporization is 186.5 kJmol-1?
Solution
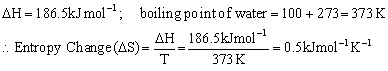
Q12. Among the enthalpies of fusion and enthalpies of freezing, which one is positive and which one is negative and why?
Solution
Melting is endothermic process, so all enthalpies of fusion are positive because ice requires heat for melting. While in freezing of water equal amount of heat is given to surrounding i.e. freezing is exothermic process. Thus all enthalpies of freezing are negative.
Q13. How does the intermolecular interaction affect the magnitude of enthalpy change during phase transformation?
Solution
The strength of intermolecular interaction determines the magnitude of enthalpy change. For example in water there is strong intermolecular interaction in the form of hydrogen bond and in acetone the intermolecular interaction is weak dipole-dipole interaction. Hence enthalpy of vaporization is greater in case of water.
Q14. For the reaction:
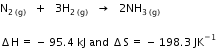 Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.
Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.
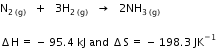 Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.
Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.Solution

Q15. Calculate the difference between heats of reaction at constant pressure and at constant volume for the reaction at 25oC in kJ. 
Solution
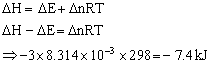
Q16. The enthalpies of formation of C2H4 (g), CO2 (g), H2O (l) at 25oC atmospheric pressure are 52, -394 and -286 kJ mol-1 respectively. Calculate the enthalpy of combustion of C2H4.
Solution
The chemical equation for the above reaction is:
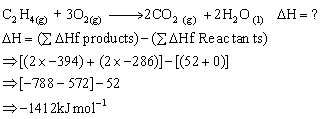
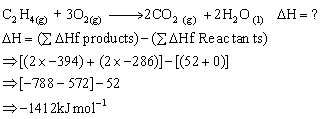
Q17. State the Zeroth law of thermodynamics.
Solution
The Zeroth law states that if two bodies A and B are in thermal equilibrium with another body C, then the bodies A and B will also be in thermal equilibrium with each other.
Q18. One mole of ethyl alcohol is completely burnt in oxygen to form water and carbon dioxide at 25oC to give 327 k cals. Calculate the heat of reaction at constant volume.
Solution
The chemical equation for this reaction is:
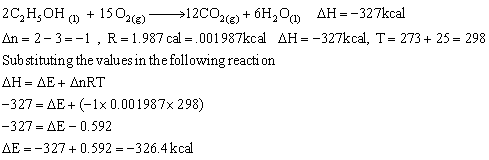
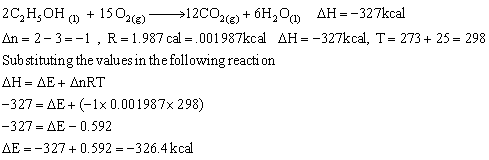
Comments
Post a Comment