Q1. If 9.006 grams of a gas are enclosed in a 50.00 liter vessel at 273.15 K and 2.000 atmospheres of pressure, what is the molar mass of the gas? What gas is this?
Solution
P = 2.000 atm
V = 50.00L
R = 0.0821 L atm mol-1 K-1
T = 273 K
We know,
 The answer (2.019 g mol-1) is approximately that of hydrogen gas, H2.
The answer (2.019 g mol-1) is approximately that of hydrogen gas, H2.
 The answer (2.019 g mol-1) is approximately that of hydrogen gas, H2.
The answer (2.019 g mol-1) is approximately that of hydrogen gas, H2.
Q2. 5.0g of an ideal gas occupies 9.2 L at STP. What volume would it occupy at 120°C and 92 mm Hg?
Solution
PV = nRT
At STP, P = 760mm Hg, T = 273 K
P1 = 760 mm
V1 = 9.2L
T1= 273K
P1= 92 mm
V2 = ?
T2 = 120 + 273 = 393K
Using relation:


Q3. The relation between Celsius scale and Fahrenheit scale of measuring temperature is
Solution
The relationship between Celsius and Fahrenheit scale is oC = 5/9 (oF–32).
Q4. The vapour pressure of water at 300 K in a closed container is 0.4 atm. If the volume of container is doubled, its vapour pressure at 300 K will be:
Solution
The vapour pressure of water will remain same as the temperature is unchanged.
Q5. Convert 25oC into Kelvin scale.
Solution
K = oC + 273.15
= 25oC + 273
= 298.15 K
Q6. Non-polar molecules do not have dipoles like polar molecules but still they form solids or liquids. Explain
Solution
Atoms of non-polar molecules are electrically symmetrical.
But unsymmetrical charge distribution takes place due to the rapid motion of electrons; a momentary accumulation of electron density occurs on one side of the atom. This causes the development of temporary instantaneous dipole on the atom.
This temporary dipole formed in one atom or molecules induces a dipole in the other atom. The temporary dipoles of both the atoms attract each other and this temporary force of attraction between two temporary dipoles is known as London force.
As a result of this force, the non-polar molecules also form solids or liquids.
Q7. What volume is needed to store 0.050 moles of helium gas at 202.6 kPa and 127ºC?
Solution
We know that, PV = nRT
P = 202.6 kPa
n = 0.050 mol
T = 127 + 273 = 400K
V = ? L
R = 8.314 J K-1 mol-1
202.6 x V = 0.050 x 8.314 x 400
202.6 V = 166.28
 V = 0.821 L or 821 mL
V = 0.821 L or 821 mL
 V = 0.821 L or 821 mL
V = 0.821 L or 821 mL
Q8. The average velocity of carbon dioxide gas at T1K and its most probable velocity at T2K is 9.0 x 104 cm sec-1. Calculate the value of T1 and T2?
Solution
Given:
The average velocity of carbon dioxide gas at T1K and its most probable velocity at T2K is 9.0 x 104 cm sec-1
Solution:
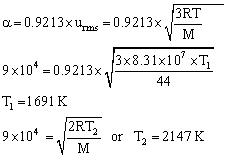
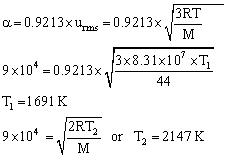
Q9. A mixture of gases contains H2 and O2 gases in the ratio of 1:4 (w/w). What is the molar ratio of the two gases in the mixture?
Solution
Given:
The ratio of the weights of the gases H2 and O2 are 1:4 (w/w)
Solution:
Molar masses of H2 and O2 are 4 and 32, respectively.
Hence, the mole ratio is as follows:


Q10. How does the molecular shape of a molecule affect the strength of Dispersion forces?
Solution
The shapes of the molecules affect the strength of dispersion forces.
Long thin molecules can develop bigger temporary dipoles due to electron movement than the short molecules containing same number of electrons.
Long thin molecules also lie closer together.
For example butane and 2-methyl propane has a molecular formula C4H10 but the atoms are arranged differently in both. In butane the carbon atoms are arranged in a single chain, but 2-methyl propane is a shorter chain with a branch.
So butane has a higher boiling point because the dispersion forces are greater.
The molecules are larger so they set up temporary dipole and can lie closer together than the shorter and fatter 2-methyl propane molecules.
Q11. Which of the following sets consists of gases with same rate of diffusion:
Solution
Gases with same vapour density should have same rate of diffusion.
Q12. A gas tanker carries helium gas at a pressure of 2.5 atmospheres at 25oC. The tanker can withstand a maximum pressure of 10 atmospheres. It collides with a truck and catches fire. According to the above information the tanker will blow up after the collision or it will catch fire. Explain. (Melting point of iron - 1535oC)
Solution
Given:
A gas tanker carries helium gas at a pressure of 2.5 atmospheres at 25oC.
The tanker can withstand a maximum pressure of 10 atmospheres.
The pressure built up in the tanker at melting point of iron is: P1 = 2.5 atm, P2 = ? , T1 = 25oC, T2 = 1535oC = 1808 K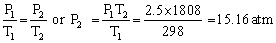 Since, the pressure of the gas in the tank is much more than 10 atm at the melting point, the tank will blow up before reaching the melting point.
Since, the pressure of the gas in the tank is much more than 10 atm at the melting point, the tank will blow up before reaching the melting point.
Q13. What is Capillary action Explain?
Solution
Capillary action is the ability of a substance to flow up in a narrow tube against the force of gravity. It results from the combination of two forces the attraction force between molecules in a liquid called cohesive force and the attraction between the molecules in a liquid and the molecules of the surface of the tube called as adhesive force.
Q14. Give reasons for the following: (i) Glycerine is more viscous than water (ii) Evaporation causes cooling
Solution
(i) Glycerine has stronger intermolecular forces than water hence, it is more viscous than water.
(ii) Evaporation causes cooling because the molecules that undergo evaporation are high energy molecules so the kinetic energy of molecules which are left behind is less. Since the average kinetic energy of the remaining molecules is less the temperature of these molecules is also lower. If temperature is kept constant the distribution of kinetic energy will be same. The high energy molecules keep on escaping from the liquid into the gas phase. This phenomenon brings a cooling effect.
Q15. What is the freezing and boiling points of water at 1 atm pressure in Fahrenheit scale?
Solution
The freezing and boiling points of water at 1 atm are 32oF and 212oF respectively according to the Fahrenheit scale.
Q16. If a graph is plotted between volume V v/s temperature oC at constant pressure. At what temperatures will it cut the volume and temperature axis?
Solution
The graph cuts the volume axis at 0oC and the temperature axis at -273.15 oC, which is also known as the absolute temperature.
Q17. Why do the falling liquid drops assume a spherical shape?
Solution
Liquid drop always assume a spherical shape. This is because drop always tends to acquire minimum surface area due to the surface tension, and for a given volume, the surface area of sphere is minimum.
Q18. If the absolute temperature of V mL of a gas is doubled and the pressure reduced to one half, its new volume will be
Solution

Q19. How gases can be liquefied?
Solution
Gases can be liquefied by compressing it and by decreasing the thermal energy of their molecules by lowering the temperature.
Q20. A student was given Water, Benzene, Orange juice, and Glycerol to pipette out in four different beakers. The liquid that will be relatively difficult to be sucked into the pipette will be:
Solution
Among the given liquids Glycerol has the highest viscosity so it is relatively difficult to be sucked into the pipette.
Q21. A sample of gas is found to occupy a volume of 900cm3 at 27oC. Calculate the temperature at which it will occupy a volume of 300cm3, provided the pressure is kept constant?
Solution
Given:
V1 = 900 cm3
V2 = 300 cm3
T1 = (27 + 273) K = 300 K, T2 = ?
Applying Charles law, we get,
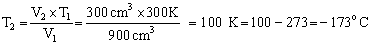
Q22. Arrange the following forces in increasing order of their strength - Dipole-dipole interaction, hydrogen bond and dispersion forces.
Solution
Hydrogen bond > Dipole-dipole interaction > Dispersion force
Q23. A gas cylinder contains air at a pressure of 15 bars at 20oC. The cylinder is provided with a safety valve which can withstand a pressure of 35 bars. Calculate the temperature to which the tank can be safely heated?
Solution
Given:
P1=15 bar P2 = 35 barT1 = 20oC = 293 KT2 = ?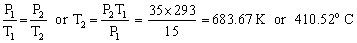 The safety valve will blow up when the temperature will exceed 683.67 K.
The safety valve will blow up when the temperature will exceed 683.67 K.
Q24. What types of intermolecular forces exist between the molecules of acetic acid (CH3COOH) and carbon tetrachloride (CCl4)?
Solution
In acetic acid (CH3COOH), hydrogen bonding, dipole-dipole interactions and dispersion force are present whereas in carbon tetrachloride (CCl4) only dispersion non-polar forces are present.
Q25. Out of fluorine and nitrogen monoxide which one has the higher melting and boiling points? Explain.
Solution
Nitrogen monoxide has higher melting and boiling points because nitrogen monoxide is a polar molecule with the strong Dipole-Dipole attraction forces.
Q26. The change in the average kinetic energy of neon atoms when the temperature is raised from 20oC to 40oC is
Solution
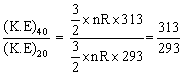
Q27. Knowledge of Z helps us to calculate:
Solution
Knowledge of Z helps us to calculate the exact volume of the real gas by using the relation,


Q28. Which one has the highest boiling point CHCl3 or CCl4?
Solution
The molecule of CHCl3 contains three chlorine atoms which are highly electronegative and have strong Dipole-dipole attractions. Hence, it is is highly polar.
CCl4 molecule has uniform partial negative charge in all directions. Hence, it is non-polar.
The molecule of CCl4 is also bigger than CHCl3 so it has a strong dispersion force which leads to higher boiling point than CHCl3.

Q29. Give reason for the high compressibility of gases on the basis of kinetic molecular theory?
Solution
According to the kinetic molecular theory, high compressibility of gases is due to large empty spaces between the molecules.
Q30. What is the relationship between three types of molecular speeds at a given temperature?
Solution
The three type of molecular speeds which are Most probable speed, Average speed and Root mean square speed are related as  at a given temperature.
at a given temperature.
 at a given temperature.
at a given temperature.
Q31. At what pressure would 0.450 mole of nitrogen gas at 23.0 °C occupy 8.90 L?
Solution
 P =?
V = 8.90 L
n = 0.450 mol
R = 0.0821 L atm mol-1 K-1
T = 23 + 273 = 296 K
P =?
V = 8.90 L
n = 0.450 mol
R = 0.0821 L atm mol-1 K-1
T = 23 + 273 = 296 K

Q32. A flask containing 250 mg of air at 27oC is heated till 25% of air is expelled from it. Calculate the final temperature of the gas?
Solution
Let the Volume of the flask be V and final temperature be T (K)
So Mass of air expelled at T (K) =
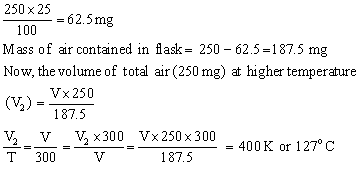 Therefore, the final temperature of the gas will be 400 K or 127oC.
Therefore, the final temperature of the gas will be 400 K or 127oC.
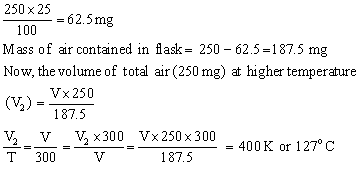 Therefore, the final temperature of the gas will be 400 K or 127oC.
Therefore, the final temperature of the gas will be 400 K or 127oC.
Q33. Why do gases deviate from ideal behaviour?
Solution
The behavior of real gases usually agrees with the predictions of the ideal gas equation to within 5% at normal temperatures and pressures. At low temperatures or high pressures, real gases deviate significantly from ideal gas behavior.
Real gases deviate from ideal gas law because their molecules interact with each other. At high pressure the molecules of gases are very close to each other so the molecular interactions start operating and these molecules do not strike the walls of the container with full impact. Thus the pressure exerted by the gas is lower than the pressure exerted by the ideal gas. At high pressure the repulsive forces also come into action .So the constants of pressure and volume are corrected and the equation is written as:
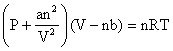 This equation is known as van der Waals equation.
This equation is known as van der Waals equation.
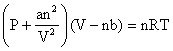 This equation is known as van der Waals equation.
This equation is known as van der Waals equation.
Q34. A student filled a balloon with hydrogen at room temperature. The balloon will burst if pressure exceeds 0.2 bars. If at 1 bar pressure the gas occupies 0.27L volume; up to what volume can the balloon be expanded?
Solution
Given:
P1 = 1 bar 2 = 0.2 bar V1 = 0.27 L V2 = ? According to Boyles law, we get, P1V1 = P2V2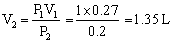 Up to 1.35 L volume the balloon can be expanded.
Up to 1.35 L volume the balloon can be expanded.
Q35. A sample of ideal gas occupies a volume of 238 mL at STP. To what temperature must the sample be heated if it is to occupy a volume of 185 mL at 2.25 atm?
Solution
At STP, P= 1 atm, T = 273 K
P1 = 1
V1 = 238 mL
T1= 273K
P1= 2.25 atm
V2 = 185 mL
T2 = ?
Using relation:


Q36. Calculate the pressure of 2 mol of ammonia at 27oC when its volume is 5 litres according to van der Waals equation? (Given that a = 4.17, b = 0.03711)
Solution
Given:
n = 2, V = 5 litres
T = 273 + 27 = 300 K
R = 0.082 lit atm deg-1 mole-1
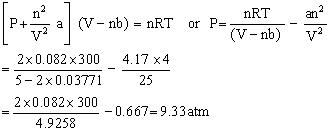
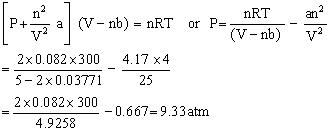
Q37. Which substances among the following experiences dipole-dipole intermolecular forces?
SiF4, CHCl3, CO2, SO2
Solution
SO4 and CHCl3 experience dipole-dipole intermolecular forces.
Q38. What is Clausius-Clapeyron equation and what is its importance?
Solution
The following equation is known as Clausius-Clapeyron equation
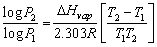 The Clausius – Clapeyron equation is the relationship between Vapour pressure and temperature. It gives a relationship between the natural log of vapour pressure and the inverse of temperature. It is a convenient way to measure the heat of vaporization in laboratory and to measure vapour pressure of a liquid at a temperature if the heat of Vaporization and Vapour pressure at one temperature are known
The Clausius – Clapeyron equation is the relationship between Vapour pressure and temperature. It gives a relationship between the natural log of vapour pressure and the inverse of temperature. It is a convenient way to measure the heat of vaporization in laboratory and to measure vapour pressure of a liquid at a temperature if the heat of Vaporization and Vapour pressure at one temperature are known
Q39. Calculate the pressure of 1 x 1021 molecules of nitrogen dioxide when enclosed in a vessel of capacity of 2.5 L capacity at temperature 27ºC?
Solution
 Now, PV = nRT
P = ? atm
V = 2.5 L
R = 0.0821 L atm mol-1 K-1
T = 27 + 273 = 300 K
n = 1.66 x 10-3 mol
Now, PV = nRT
P = ? atm
V = 2.5 L
R = 0.0821 L atm mol-1 K-1
T = 27 + 273 = 300 K
n = 1.66 x 10-3 mol
 P = 1.6 35 x 10-2 atm
P = 1.6 35 x 10-2 atm
Q40. Why does mercury have concave-downward meniscus?
Solution
In mercury, the cohesive forces are stronger than adhesive forces. Therefore, mercury have concave-downward meniscus.
Q41. A closed gas system initially has pressure and temperature of 86.6torr and 424K with the volume unknown. If the same closed system has values of 597torr, 1240mL and 455K, what was the initial volume in mL?
Solution
P1 = 86.6 torr
V1 = ?
T1= 424K
P1= 597 torr
V2 = 1240 mL
T2 = 455K
Using relation:






Comments
Post a Comment