Q1. Which technique one can employ to separate crude oil in petroleum industry?
Solution
Fractional distillation technique can be used to separate crude oil in petroleum industry.
Q2. How to detect percentage of oxygen in an organic compound?
Solution
Percentage of oxygen in an organic compound is detected by the difference between the total percentage and the sum of percentages of all other elements present.
Q3. Alkyl radical stability increases as we proceed from:
Solution
Alkyl radical stability increases as we proceed from Methyl free radical<Ethyl free radical< Isopropyl free radical as more hyperconjugating structures are formed as we proceed from methyl to Isopropyl.
Q4. What are the three steps in the halogenation reaction?
Solution
Three steps in halogenation reaction are initiation, propagation and termination.
Q5. What is chain isomerism? Give example.
Solution
Two or more compounds which have a similar molecular formula but different arrangement of carbon atoms in straight or branched chains are referred to as chain isomers, and the phenomenon is known as chain isomerism. For example C5H12 represents three compounds.

Q6. What are the common techniques used for purification of organic compounds?
Solution
The common techniques used for purification are: Sublimation, Crystallisation, Distillation, Differential extraction and Chromatography.
Q7. What is a homolytic cleavage?
Solution
In hemolytic cleavage one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms. Thus in this movement of a single electron takes place instead of an electron pair.
Q8. How will you test for phosphorus in an organic compound?
Solution
The organic compound is heated with an oxidising agent. The phosphorus present in the compound is oxidized to phosphate. The solution is boiled with nitric acid and then treated with ammonium molybdate . A yellow colouration indicated presence of phosphorus.

Q9. What is the Prussian blue color due to in the test for nitrogen?
Solution
In the test of nitrogen the Prussian blue color formed is of ferriferrocyanide.
Q10. What are the different types of structural isomers?
Solution
Different types of structural isomers are chain isomerism, position isomerism, functional group isomerism and metamerism.
Q11. Discuss the order of stability of the carbocation

Solution
Carbications are highly unstable and reactive species. Alkyl groups directly attached to the positively charged carbon stabilize the carbocation due to inductive and hyperconjugation effects. So the order of stability of the carbocation is:

Q12. What are optically active and optically inactive compounds?
Solution
When plane polarized light is passed through a substance, it may or may not rotate the plane of the plane polarized light. The substance which does not rotate the plane of the plane polarized light is known as optically inactive compound, while a substance which rotates the plane of the plane polarized light is known as optically active substance.
Q13. Which of the following ions is most stable?
Solution
Tertiary carbonium ions are most stable due to hyperconjugation effect and +I effect of methyl group.
Q14. Process of separation of mixtures into their components and to purify the compounds by using adsorption is known as:
Solution
Process of separation of mixtures into their components and to purify the compounds is known as chromatography.
Q15. Rate of reaction of alkanes with halogens is:
Solution
Rate of reaction of alkanes with halogens is F2>Cl2> Br2> I2.
Q16. What is Lassaigne's test?
Solution
Nitrogen, sulphur, halogens and phosphorus present in an organic compound are detected by Lassaignes test. The elements present in the compound are converted from covalent form to ionic form by fusing the compound with sodium metal.

Q17. What is the order of rate of reaction of alkanes with halogens F2, Cl2, Br2 and I2?
Solution
The order of rate of reaction of alkanes with halogens is F2 > Cl2 > Br2 > I2.
Q18. What is lead-sulphide test for sulphur?
Solution
The sodium fusion extract is acidified with acetic acid and lead acetate is added to it. A black precipitate of lead sulphide indicates the presence of sulphur.
S2- + Pb2+ → PbS
Black
Q19. Discuss the shape of CH3+ ion?
Solution
The shape of methyl carbonium ion is derived from the overlap of three equivalent C(sp2) hybridized orbitals with 1s orbital of each of the three hydrogen atoms. Each bond may be represented as C(sp2)-H(1s) sigma bond. The remaining carbon orbital is perpendicular to the molecular plane and contains no electrons.
 Shape of methyl cation
Shape of methyl cation
Q20. 
Solution
Q21. What is Metamerism?
Solution
Metamerism arises due to different alkyl chains on either side of the functional group in the molecule.
Q22. What is fractional distillation process?
Solution
When the difference in boiling points of two liquids is not much, simple distillation cannot be used to separate them. The vapours of such liquids are formed within the same temperature range and are condensed simultaneously. The technique of fractional distillation is used in such a process.
Q23. In sulphur estimation, 0.157 g of an organic compound gave 0.4813 g of barium sulphate. The percentage of sulphur in the compound is:
Solution
Molecular mass of BaSO4 = 137+32+64 = 233 g 233 g BaSO4 contains 32 g sulphur 0.4813 g BaSO4 contains = 32 X 0.4813/ 233 g sulphur Percentage of sulphur=32X 0.4813 X100/ 233 X 0.157 = 42.10%
Q24. What is the violet color due to, when sodium fusion extract is treated with sodium nitroprusside in the test for sulphur?
Solution
On treating sodium fusion extract with sodium nitroprusside, appearance of a violet color due to formation of [Fe(CN)5NOS]4- indicates the presence of sulphur.
Q25. How can you separate aniline from aniline-water mixture?
Solution
Steam distillation technique is applied to separate substances which are steam volatile and are immiscible with water like aniline from aniline-water mixture.
Q26. What is adsorption chromatography?
Solution
Adsorption chromatography is based on the fact that different compounds are adsorbed on an adsorbent to different degrees. Commonly used adsorbents are silica gel and alumina. When a mobile phase is allowed to move over a stationary phase the components of a mixture move by varying distances over the stationary phase.
Q27. What is crystallization process?
Solution
This is a technique for purification of solid organic compounds. It is based on the difference in the solubilities of the compound and the impurities in a suitable solvent.The impure compound is dissolved in a solvent in which it is sparingly soluble at room temperature. The solution is concentrated to get a nearly saturated solution.On cooling the solution pure compound crystsllises outand is removed by filtration.
Q28. Write the position isomers of C3H8O.
Solution
C3H8O represents following position isomers.

Q29. How can be covalent bonds cleaved?
Solution
Covalent bonds can be cleaved by homolytic and heterolytic cleavage.
Q30. What is the electrophilic centre in the molecule CH3CN?
Solution
In the molecule CH3CN, the starred carbon atom is the electrophilic centre as has partial positive charge due to polarity of bond.

Q31. What is Rf value?
Solution
Distance moved by the substance from the base line to the distance moved by the solvent from the base line is known as the Rf value.
Q32. When nitrogen and sulphur are both present in an organic compound, what is the test to perform?
Solution
When nitrogen and sulphur are both present in an organic compound, sodium thiocyanate is formed and it gives blood red colour.
Na + C + N+ S 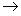 NaSCN
Fe3+ + SCN -
NaSCN
Fe3+ + SCN - 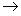 [ Fe (SCN) ]2+
Blood red
[ Fe (SCN) ]2+
Blood red
Q33. The correct representation of 4-hydroxy-2-methylpent-2-en-1-al is:
Solution
The correct representation of 4-hydroxy-2-methylpent-2-en-1-al is:

Q34. Categorise the following ions as nucleophile or electrophile? HS-, BF3, NO2+, C2H5O-, NH2-
Solution
Nucleophile among the following are : HS-, C2H5O-, NH2- Electrophile among the following are : BF3, NO2+
Comments
Post a Comment