Q1. Generally atomic radii increases down the group, but atomic radius of Ga is less than that of Al. Why?
Solution
The presence of additional 10 d-electrons offer only poor screening effect for the outer electrons from the increased nuclear charge in gallium hence the atomic radius of Ga is less than Al.
Q2. What are the applications of silicon compounds?
Solution
Applications of Silicon Compounds:a. Ferro-silicon and calcium silicate are used as alloying elements in the development of steel or cast iron.b. Silicon carbide possess a diamond like crystalline structure. Due to its hardness it is used as an abrasive. c. CaSiO3 is used as a component of cement.
Q3. What are the similarities between graphite and diamond?
Solution
Both graphite and diamond are forms of carbon. As such, they are said to be allotropes of carbon.
Both occur naturally. Both are mined for industrial purposes, though larger diamonds are sought and used for other things.
Both are produced in the earth in geothermal processes.
Both can be made artificially.
Both are normally solids and highly stable. And they are both difficult to burn, even in an oxygen environment.
Q4. Give the type of hybridization involved in BCl3NH3.
Solution
sp3hybridization.
Q5. Suggest reasons why the B–F bond lengths in BF3 (130 pm) and BF4- (143 pm) differ.
Solution
In BF3, B atom is sp2 hybridised and has one unhybridised empty 2p atomic orbital.
This empty 2p orbital overlaps sideways with fully filled 2p atomic orbital of F to form dative pΠ- pΠ bond.
This reduces the bond length.
In BF-4, B atom is sp3 hybridised, all B-F bond are single covalent bond.
Q6. Give any two uses of silicones.
Solution
Uses of silicones:
Silicone oils are used for high temperature oil baths, high vacuum pumps and low temperature lubricantion.
They are used as an insulating materials for electric motors and other electrical appliances.
Q7. Draw the structure of diborane, boron trichloride and boric acid.
Solution
Q8. If B–Cl bond has a dipole moment, explain why BCl3 molecule has zero dipole moment.
Solution
B-Cl has a dipole due to the difference in the electronegativity of boron and chlorine atom.
The overall dipole of a molecule also depends on the geometry.
The geometry of BCl3 is planar with a bond angle of 120 degree.
The resultant dipole of two B-Cl bonds cancels the third one, resulting in net zero dipole.
Q9. Write reactions to justify amphoteric nature of aluminium.
Solution
Amphoteric nature means aluminium shows both acidic and basic character.
Reaction with acids:
2Al(s) + 6HCl (aq) → 2Al3+ (aq) + 6Cl– (aq) + 3H2 (g)
Reaction with Alkalis:
2Al (s) + 2NaOH (aq) + 6H2O (l) → 2 Na+ [Al(OH)4] –(aq) + 3H2(g)
Sodium
Tetrahydroxoaluminate (III)
Q10. Explain what happens when boric acid is heated.
Solution
Orthoboric acid when heated above 370K forms metaboric acid, HBO2 which on further heating yields boric oxide, B2O3.

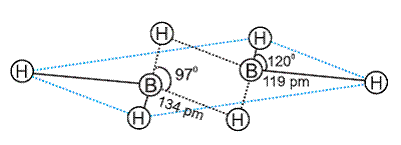
Q11. Explain structures of diborane.
Solution
In the structure of diborane, the four terminal hydrogen atoms and the two boron atoms lie in one plane.
Above and below this plane, there are two bridging hydrogen atoms.
The four terminal B-H bonds are regular two centre-two electron bonds while the two bridge (B-H-B) bonds are different and can be described as three centre -two electron bonds or banana bond.

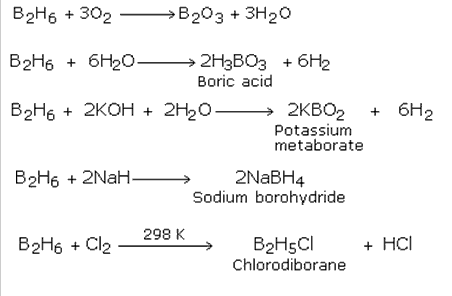
Q12. Give one reaction for the preparation of orthoboric acid.
Solution
Na2B4O7 + 2HCl + 5H2O → 2NaCl + 4B(OH)3
Boric acid
Q13. What are silicones?
Solution
These are organosilicon polymers containing Si-O-Si linkages.
They are formed by the hydrolysis of alkyl or aryl substituted chlorosilanes and their subsequent polymerisation.
The alkyl or aryl substituted chlorosilanes are prepared by the reaction of Grignard reagent and silicon tetrachloride.
RMgCl + SiCl4 → RSiCl3 + MgCl2
Grignard reagent
R stands for -CH3 , -C2H5 or -C6H5 groups
Q14. 

Solution
Q15. Give an account of Borax bead test.
Solution
Borax on heating first loses water molecules and forms sodium metaborate which on further heating turns into a transparent liquid, which solidifies into glass like material known as borax bead.
Na2B4O7.10H2O Na2B4O7
Na2B4O7  2NaBO2 + B2O3
Sodium Boric
metaborate anhydride
The metaborates of many transition metals have characteristic colours and, therefore, borax bead test can be used to identify them in the laboratory.
2NaBO2 + B2O3
Sodium Boric
metaborate anhydride
The metaborates of many transition metals have characteristic colours and, therefore, borax bead test can be used to identify them in the laboratory.
Q16. Give any one method of preparation of silica.
Solution
Q17. Which is the most stable allotrope of carbon?
Solution
Graphite is the most stable allotrope of carbon.
Q18. The boron compounds generally behave as Lewis acid. Explain.
Solution
As the number of valence electrons in boron is three, it forms compounds which are electron deficient.
Such electron deficient molecules have tendency to accept a pair of electrons to achieve stable electronic configuration and thus, behave as Lewis acids.
Q19. What is “inorganic benzene”?
Solution
Borazine, B3N3H6 is known as “inorganic benzene”.
Q20. What makes diamond an excellent abrasive?
Solution
Diamond has a solid three dimensional structure.
Each carbon atom is present at the same distance to each of its neighboring carbon atoms.
In this rigid network atoms cannot move. This makes diamond hard.
Eventually, diamond is the hardest known natural mineral. Due to its hardness, it is used for rock drilling and for making abrasives for sharpening hard tools.
Q21. What happens when: (give reactions only) (a) Boron is heated in air (b) Boron reacts with dilute HCl
(c) Boron reacts with Chlorine
Solution
(a) Boron reacts with oxygen and nitrogen.
4B(s) + 3O2(g) 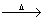 2B2O3(s)
2B2O3(s) 2B(s) + N2(g)
2B(s) + N2(g) 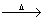 2BN(s)
(b) B(s) + Dil. HCl
2BN(s)
(b) B(s) + Dil. HCl 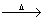 No reaction
(c) 2B(s) + 3Cl2(g)
No reaction
(c) 2B(s) + 3Cl2(g) 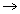 2BCl3(s)
2BCl3(s)
Q22. Why graphite is soft but diamond is hard in nature?
Solution
Graphite has layers structure. There are weak van der Waal's forces between the layers. So, the layers can slide over one another. Therefore, graphite is soft. In diamond each carbon atom is the same distance to each of its neighboring carbon atoms. In this rigid network atoms cannot move.
Q23. How is cross linked polymer of the silicones synthesized?
Solution

Q24. Aluminum can form [AlF6]− but Boron cannot form [BF6]−. Why?
Solution
Due to unavailability of vacant d-orbital as in Al, B is unable to expand its octet and thus cannot form [BF6]− as [AlF6]− .
Comments
Post a Comment