Q1. Give the structure of A and B: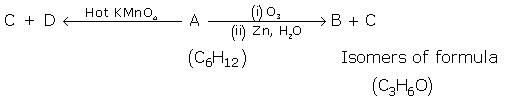
Solution

Q2. Why alkanes are also known as paraffin?
Solution
Alkanes are relatively unreactive under ordinary conditions and so they are also known as paraffins.
Q3. Is it possible to isolate pure staggered ethane or pure eclipsed ethane at room temperature?
Solution
It is not possible to isolate pure staggered form of ethane or pure eclipsed form of ethane at room temperature because of less difference in their energy they can get converted into each other very fast.
Q4. Why does ethyne show acidity?
Solution

Q5. Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example.
Solution
For preparing alkanes containing odd number of carbon atoms, a mixture of two alkyl halides has to be used. These two alkyl halides may react in three different ways producing a mixture of three alkanes instead of desired alkane. For example, Wurtz reaction between bromoethane and 1-bromopropane give the following three alkanes instead of a single alkanes of odd number of C-atoms.

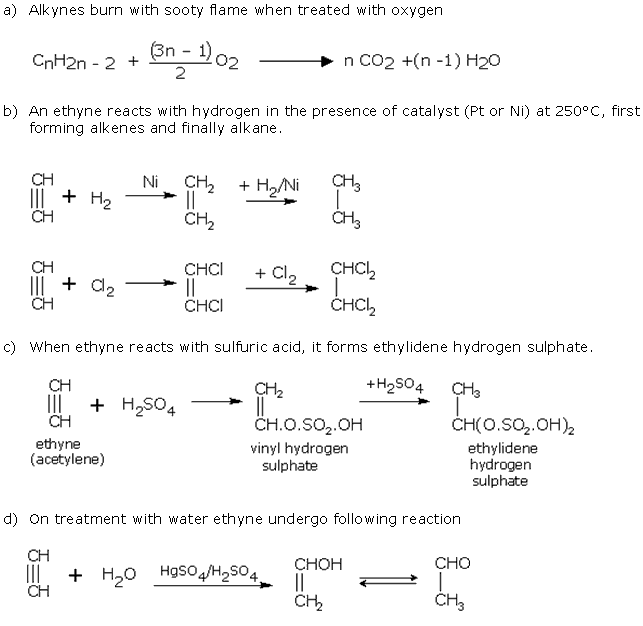
Q6. Write equations for the following chemical conditions:a) When alkynes burns in air or oxygen.b) When ethyne reacts with hydrogen in the presence of catalyst (Pt or Ni) at 250oC.c) When ethyne reacts with chlorine.d) When ethyne reacts with water in the presence of suitable reagent.
Solution
Q7. A hydrocarbon 'X' takes up two molecules of hydrogen and is converted into a saturated hydrocarbon. On ozonolysis, 'X' gives a mixture of three carbonyl compounds namely, acetaldehyde, acetone and propan-1, 3-dial. Assign structure to compounds X.
Solution
Since the compound takes up two molecules of hydrogen, it must have two double bonds. The formation of three products on ozonolysis indicates that the compounds have unsaturation at two places. The equations are:
Q8. 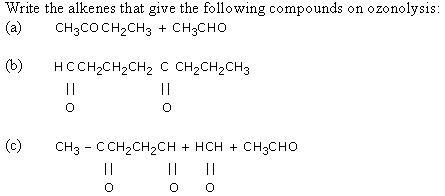
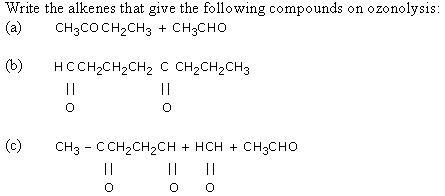
Solution
Q9. Draw all possible isomers of the alkyne with molecular formula C5H8.
Solution
Q10. How many chain isomers are possible for C4H10.Draw their structure.
Solution
2 isomers are possible for C4H10.
CH3 CH2 CH2 CH3 and CH3CH(CH3)2

Q11. 

Solution
1, 2-Dimethylbenzene1-Methylnaphthalene2, 4, 6-TrinitrotoluenePhenyl benzoateAminobenzene or Aniline
Q12. Arrange the following in increasing order of their release of energy on combustion:

Solution
Greater the number of carbon atoms with maximum hydrogen atoms (i.e., CH3 groups), greater is the heat of combustion. Thus, the increasing order of heat of combustion:(iii) < (iv) < (i) < (ii)
Q13. Discuss the polymerization reaction of alkynes.
Solution
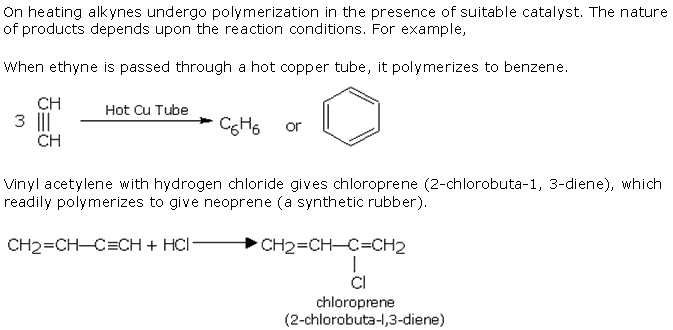
Q14. Mention the type of isomerism shown by aromatic compounds with examples.
Solution
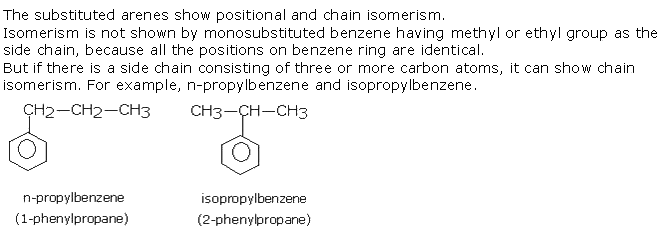
Q15. What are the harmful facts related to polynuclear hydrocarbons?
Solution
The polynuclear aromatic hydrocarbons penetrate the body rapidly and reach to the fat containing tissues.
Short-term low exposure to naphthalene may cause eye and skin irritation. If naphthalene is ingested it has the potential to cause hemolytic anaemia, a condition that involves the breakdown of red blood cells. Naphthalene is a suspected human carcinogen, and has been proven to cause damage to the kidneys and to the liver. Higher incidences of lung and skin tumors have been reported for people who have been occupationally exposed to naphthalene and other Polynuclear aromatic hydrocarbons.
An exposure to Anthracene can cause skin and eye irritation. Repeated exposure may cause alteration of skin pigments as well as cancerous growth. Inhaled anthracene can cause bronchitis like symptoms. There is limited information on human reproductive implications. Anthracene may cause genetic mutations in cells.
Phenanthrene is also a skin and eye irritant, with increasing effects in sunlight due to photosensitization. There is currently no data available for human oral and inhalation exposure. It is however, a suspected carcinogen, although there is no data for humans.
Q16. Discuss three methods to prepare benzene.
Solution
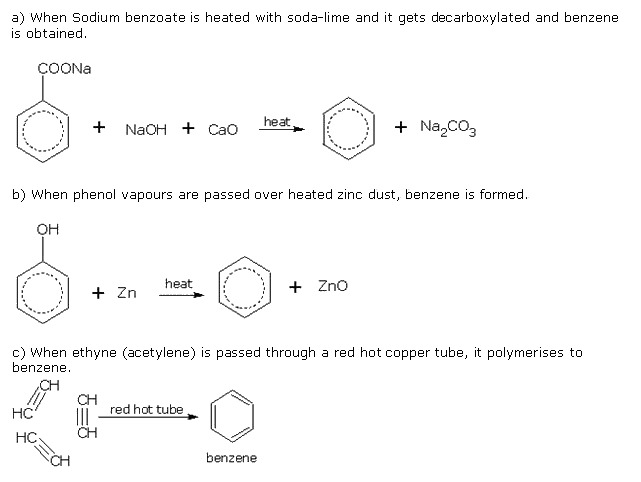
Q17. Write two tests to distinguish 1-pentene from n-pentane?
Solution
1-Pentene and n-Pantane can be distinguished by the following:i. Reaction with Br2 in CCl4 - 1-pentene will decolourise the brown colour of the solution of Br2 in CCl4. But n-Pentane will not decolourise the colour of bromine at room temperature. 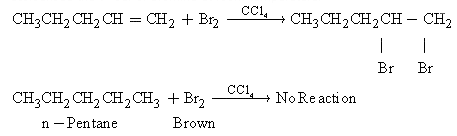 ii. Reaction with cold dilute KMnO4(Baeyer's test): 1-Pentene will react with Baeyer's reagent and its colour will be discharged. n-Pentane does not react.
ii. Reaction with cold dilute KMnO4(Baeyer's test): 1-Pentene will react with Baeyer's reagent and its colour will be discharged. n-Pentane does not react.
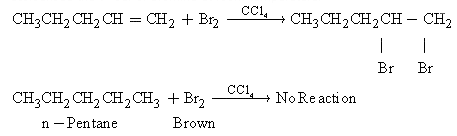 ii. Reaction with cold dilute KMnO4(Baeyer's test): 1-Pentene will react with Baeyer's reagent and its colour will be discharged. n-Pentane does not react.
ii. Reaction with cold dilute KMnO4(Baeyer's test): 1-Pentene will react with Baeyer's reagent and its colour will be discharged. n-Pentane does not react.
Q18. 

Solution
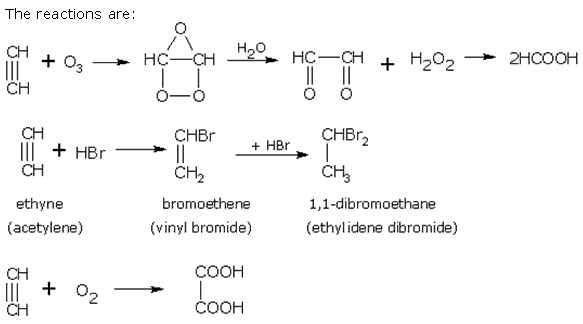
Q19. One mole of a hydrocarbon (A) reacts with one mole of bromine giving a dibromo compound, C5H10Br2. Substance (A) on treatment with cold dilute alkaline KMnO4 solution forms a compound C5H12O2. On ozonolysis (A) gives equimolar quantities of propanone and ethanal. Deduce the structural formula of (A).
Solution
One mole of the hydrocarbon (A) adds on one mole of bromine to form C5H10Br2 therefore, (A) must be an alkene having molecular formula C5H10. The position of double bond is indicated by ozonolysis as
Q20. Arrange the different type of conformation of butane in the decreasing order of stability.
Solution
Anti > Skew > Eclipsed > Fully Eclipsed
Comments
Post a Comment